Research In Practice Blog
Breadcrumb
Urinary stone disease is a common and painful condition that carries significant morbidity. Kidney stones are one of the fastest growing health conditions among children, adolescents, and young adults. The rapid increase over a short period of time has resulted in a large number of pediatric patients who require surgery to remove kidney stones with very little information available to guide selection of treatment options.
Most children and adolescents with kidney and ureteral stones receive treatment through a procedure called ureteroscopy. This is the case even though there is some uncertainty about whether ureteroscopy or shockwave lithotripsy is the better option, according to guidelines.
A new study, published in JAMA Network Open, conducted by researchers at the Children’s Hospital of Philadelphia (CHOP), along with several academic partners, evaluated the effectiveness of stone clearance and patient-reported outcomes in children and adolescents who underwent either ureteroscopy or shockwave lithotripsy. This research is part of the Pediatric KIDney Stone (PKIDS) trial, which is the largest comparative effectiveness study of surgical interventions for children and adolescents with kidney stones. CHOP founded the PKIDS Care Improvement Network in 2019, which now includes 31 sites in the United States and Canada.
Study Design and Findings: Comparing Ureteroscopy vs. Shockwave Lithotripsy
The PKIDS study (NCT04285658), a nonrandomized clinical trial initiated by researchers and integrated into the clinical care of children and adolescents undergoing kidney stone surgery, took place between March 16, 2020, and July 31, 2023, at 31 medical centers across 22 U.S. states and 1 Canadian province that are part of the PKIDS Network. See eFigure 1 below.
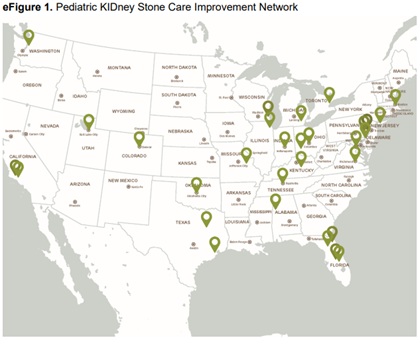
PKIDS received designation as a National Patient-Centered Clinical Research Network (PCORnet) study because it involved medical centers that contribute data to five PCORnet clinical research networks.
Researchers enrolled 1142 patients aged 8 to 21 with kidney and/or ureteral stones between 2020 and 2023 at all 31 sites in the United States and Canada.
This nonrandomized clinical trial was conducted within a clinical care setting to compare ureteroscopy and shockwave lithotripsy in children and adolescents. It yielded the following four clinically significant findings.
- First, the researchers found no meaningful differences in stone clearance between the two procedures.
- Second, compared to ureteroscopy, shockwave lithotripsy resulted in less pain and fewer urinary symptoms during the first week after the procedure.
- Third, patients who underwent shockwave lithotripsy missed less school, and their caregivers missed less work than those who had ureteroscopy.
- Finally, significantly more patients who underwent ureteroscopy (285 out of 953, or 29.9%) required a second procedure under general anesthesia to remove a ureteral stent, compared to those who received shockwave lithotripsy (6 out of 189, or 3.2%).
Researchers concluded that this evidence, derived from contemporary clinical care, raises questions about current clinical practices, where most children and adolescents with kidney stones undergo ureteroscopy.
What’s Next? Informing Future Clinical Practice Guidelines
The PKIDS trial demonstrated that ureteroscopy and shockwave lithotripsy are equally effective in removing kidney stones. However, patients who undergo shockwave lithotripsy experience a quicker recovery, less pain, and fewer urinary symptoms.
Researchers have pinpointed key factors that clinicians should consider when assessing how this study's findings should influence their practice. By actively involving patients and caregivers in the study's design, the results ensure that the outcomes are not only relevant but also vital to those they directly impact. The study highlighted the impacts of ureteroscopy and shockwave lithotripsy on physical, emotional, and social health, as well as their effects on patients' ability to return to school and work—information that was previously unknown.
Furthermore, the results represent a diverse group of children and adolescents with kidney stones who were treated at medical centers known for their expertise in pediatric kidney stone care. Clinicians can utilize these findings to improve the selection process for shockwave lithotripsy or ureteroscopy and to better guide patients regarding what to expect during their postoperative experiences.
"Our findings offer critical insights that can pave the way for tailored treatment options for children with kidney stones and their families,” said Gregory E. Tasian, MD, MSc, MSCE, Director of the PKIDS network and an attending pediatric urologist in the Division of Urology, and Associate Director for Clinical Trials at Clinical Futures at Children's Hospital of Philadelphia. “The success of future clinical trials will be vital, and it is our hope that new clinical practice guidelines will prioritize patient-centered outcomes.”
This study was supported through a Patient-Centered Outcomes Research Institute (PCORI) funding Award (CER-2018C3-14778).
For more information, please read this CHOP news release.
Additional information and related work:
• PKIDS Care Improvement Network
• CHOP and Vanderbilt Researchers to Lead $7.37 Million Kidney Stone Research
• Study Shows Climate Change Will Lead to Increase in Kidney Stones
• New USDHub Will Create Robust Data Resource to Advance Kidney Stone Research
• CHOP Researchers Awarded NIH Grant to Create USDHub to Support Kidney Stone Researchers for Patients of All Ages
Clinical Futures author(s): Gregory Tasian, MD, MSc, MSCE, Brian Augelli, BS, Jing Bi-Karchin, PhD
Citation:
Tasian et al. “Ureteroscopy versus Shockwave Lithotripsy to Remove Kidney Stones in Children and Adolescents: A Nonrandomized Clinical Trial.” JAMA Netw Open. Online August 7, 2025. DOI: 10.1001/jamanetworkopen.2025.25789.
